## Why is Nitrogen in the Atmosphere Not Used by Plants and Animals? Short Response Explained
Have you ever wondered why, despite the atmosphere being nearly 80% nitrogen, plants and animals can’t simply absorb it and use it directly? This is a crucial question in understanding the nitrogen cycle, a fundamental process that sustains life on Earth. This article delves into the reasons behind this limitation, exploring the complex processes required to make nitrogen usable for living organisms. We’ll unravel the science behind nitrogen fixation, the various ways nitrogen becomes bioavailable, and the critical role this plays in ecosystems worldwide. We will also explore the importance of nitrogen in various products and services, analyze the advantages and benefits, and do an in-depth review of the concept. This is your comprehensive guide to understanding why atmospheric nitrogen needs a helping hand before it can nourish life.
### Understanding the Unreactivity of Atmospheric Nitrogen
The primary reason plants and animals cannot directly utilize atmospheric nitrogen (N2) lies in its molecular structure. Nitrogen exists as a diatomic molecule held together by a triple bond (N≡N). This triple bond is exceptionally strong and requires a significant amount of energy to break. This high bond energy makes atmospheric nitrogen largely unreactive under normal biological conditions.
* **The Triple Bond:** The key factor is the triple bond between the two nitrogen atoms. It’s one of the strongest bonds in nature, requiring substantial energy to break and separate the nitrogen atoms. Think of it like a sturdy bridge; it takes a lot of force to dismantle it.
* **Lack of Biological Machinery:** Plants and animals lack the necessary enzymes or biochemical pathways to directly break this triple bond and incorporate nitrogen into their organic molecules. They simply don’t have the tools to do it.
* **Inert Nature:** Due to its strong bond, atmospheric nitrogen is relatively inert. This means it doesn’t readily react with other elements or compounds. This stability is beneficial for maintaining a stable atmosphere, but it presents a challenge for life.
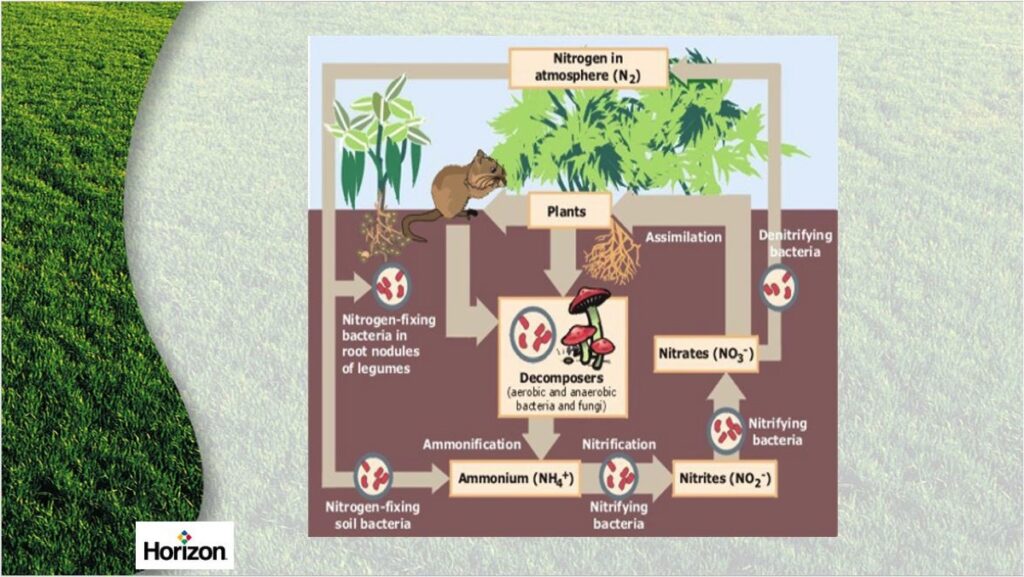
### The Nitrogen Cycle and Nitrogen Fixation
To become usable by plants and animals, atmospheric nitrogen must undergo a process called nitrogen fixation. Nitrogen fixation is the conversion of atmospheric nitrogen (N2) into ammonia (NH3) or other nitrogen compounds that plants can absorb and assimilate.
* **Biological Nitrogen Fixation:** This is the most significant pathway. Certain bacteria, primarily those living in symbiotic relationships with legumes (like soybeans, peas, and clover) or free-living bacteria in the soil and aquatic environments, possess the enzyme nitrogenase. Nitrogenase catalyzes the reduction of N2 to NH3.
* *Symbiotic Nitrogen Fixation:* In symbiotic relationships, the bacteria colonize the roots of legumes, forming nodules. The plant provides the bacteria with carbohydrates for energy, and the bacteria, in turn, provide the plant with fixed nitrogen.
* *Free-Living Nitrogen Fixation:* Free-living bacteria, such as *Azotobacter* and *Clostridium*, also fix nitrogen, contributing to soil fertility.
* **Abiotic Nitrogen Fixation:**
* *Industrial Nitrogen Fixation:* The Haber-Bosch process is an industrial method used to produce ammonia fertilizer. This process uses high pressure and temperature, along with a catalyst, to convert atmospheric nitrogen and hydrogen into ammonia.
* *Atmospheric Fixation:* Lightning strikes can provide enough energy to break the nitrogen triple bond and allow nitrogen to react with oxygen to form nitrogen oxides (NOx). These oxides are then carried to the earth’s surface in precipitation and converted to nitrates.
### How Plants and Animals Obtain Nitrogen
Once nitrogen is fixed into a usable form (ammonia, ammonium, or nitrate), plants can absorb it through their roots. Animals obtain nitrogen by consuming plants or other animals. The nitrogen is then incorporated into proteins, nucleic acids, and other essential biomolecules.
* **Plant Uptake:** Plants primarily absorb nitrogen in the form of nitrate (NO3-) and ammonium (NH4+). These ions are transported through the plant’s vascular system and used to synthesize amino acids and other nitrogen-containing compounds.
* **Animal Consumption:** Animals obtain nitrogen by consuming plants or other animals. The nitrogen-containing compounds in their diet are broken down into amino acids, which are then used to build their own proteins and other essential molecules.
### The Importance of Nitrogen in Biological Systems
Nitrogen is an essential element for all living organisms. It is a key component of:
* **Proteins:** Proteins are essential for building and repairing tissues, catalyzing biochemical reactions (enzymes), and transporting molecules.
* **Nucleic Acids:** Nitrogen is a crucial component of DNA and RNA, the molecules that carry genetic information.
* **Chlorophyll:** Chlorophyll, the pigment that allows plants to capture sunlight for photosynthesis, contains nitrogen.
### The Role of Nitrogen in Fertilizer Production: The Haber-Bosch Process
Since plants cannot directly utilize atmospheric nitrogen, the Haber-Bosch process is critical to modern agriculture. This process, developed in the early 20th century, allows the large-scale production of ammonia fertilizer. While essential for food production, the Haber-Bosch process has significant environmental consequences, including greenhouse gas emissions and water pollution.
* **The Process:** The Haber-Bosch process combines atmospheric nitrogen with hydrogen (typically derived from natural gas) under high pressure and temperature, using an iron catalyst to produce ammonia.
* **Impact on Agriculture:** This process has revolutionized agriculture, allowing for increased crop yields and supporting a growing global population.
* **Environmental Concerns:** The Haber-Bosch process is energy-intensive and contributes to greenhouse gas emissions. The overuse of nitrogen fertilizers can also lead to water pollution, including eutrophication (excessive nutrient enrichment) of aquatic ecosystems.
### Denitrification: Returning Nitrogen to the Atmosphere
The nitrogen cycle is a closed loop. Denitrification is the process by which nitrates are converted back into atmospheric nitrogen. This process is carried out by denitrifying bacteria under anaerobic conditions.
* **The Process:** Denitrifying bacteria use nitrate as an electron acceptor in the absence of oxygen, converting it to nitrogen gas (N2) or nitrous oxide (N2O), which is then released into the atmosphere.
* **Balancing the Cycle:** Denitrification plays a critical role in balancing the nitrogen cycle, preventing the accumulation of excess nitrogen in the soil and water.
## Product/Service Explanation Aligned with Why is Nitrogen in the Atmosphere Not Used by Plants and Animals? Short Response
In the context of understanding why atmospheric nitrogen is unusable by plants and animals in its raw form, a relevant product/service to consider is **Nitrogen Fertilizer**. While it’s not a direct solution to the unreactivity of N2, it addresses the consequence: the need for bioavailable nitrogen for agriculture. Nitrogen fertilizers, specifically those produced via the Haber-Bosch process, provide plants with nitrogen in a form they can readily absorb (primarily as nitrates and ammonium).
From an expert viewpoint, nitrogen fertilizer is a double-edged sword. It’s a crucial tool for modern agriculture, significantly boosting crop yields and enabling us to feed a growing global population. However, its production and use have significant environmental impacts, including greenhouse gas emissions, water pollution, and soil degradation.
The core function of nitrogen fertilizer is to supplement the limited natural nitrogen fixation processes in the soil, ensuring that plants have sufficient access to this essential nutrient for growth and development. Without it, many crops would struggle to thrive, leading to lower yields and potential food shortages.
Nitrogen fertilizer stands out due to its ability to rapidly increase the availability of nitrogen in the soil. Unlike natural processes like biological nitrogen fixation, which can be slower and more dependent on environmental conditions, nitrogen fertilizer provides an immediate boost of readily available nitrogen.
## Detailed Features Analysis of Nitrogen Fertilizer
Let’s examine the key features of nitrogen fertilizer, focusing on how they relate to the challenge of nitrogen bioavailability and the overall nitrogen cycle:
1. **Nitrogen Content (High Concentration):**
* *What it is:* Nitrogen fertilizers contain a high concentration of nitrogen, typically in the form of ammonium (NH4+), nitrate (NO3-), or urea (CO(NH2)2).
* *How it works:* When applied to the soil, these compounds dissolve and release nitrogen ions that plants can readily absorb through their roots.
* *User Benefit:* Farmers can quickly increase the nitrogen levels in their soil, promoting rapid plant growth and higher yields. Demonstrates expertise by providing a direct, efficient solution to nitrogen deficiency.
* *Demonstrates Quality:* High nitrogen content ensures that plants receive an adequate supply of this essential nutrient, leading to healthier and more productive crops.
2. **Various Forms (Ammonium, Nitrate, Urea):**
* *What it is:* Nitrogen fertilizers are available in different chemical forms, each with its own properties and application methods.
* *How it works:* Ammonium-based fertilizers are converted to nitrate in the soil through nitrification. Nitrate is highly mobile and readily absorbed by plants. Urea is converted to ammonium in the soil.
* *User Benefit:* Farmers can choose the fertilizer form that best suits their soil type, crop requirements, and application equipment. Demonstrates flexibility and adaptability to different farming practices.
* *Demonstrates Quality:* Offering various forms allows for optimized nitrogen uptake based on specific crop needs and environmental conditions.
3. **Controlled Release Options:**
* *What it is:* Some nitrogen fertilizers are formulated with a coating or other mechanism to control the rate at which nitrogen is released into the soil.
* *How it works:* The coating gradually breaks down, releasing nitrogen over an extended period. This reduces the risk of nitrogen loss through leaching or volatilization.
* *User Benefit:* Reduces the need for frequent fertilizer applications, saves time and labor, and minimizes environmental impact. Demonstrates a focus on sustainability and efficiency.
* *Demonstrates Quality:* Controlled release minimizes nitrogen losses, ensuring that plants receive a steady supply of nitrogen throughout their growth cycle.
4. **Ease of Application:**
* *What it is:* Nitrogen fertilizers are typically available in granular or liquid form, making them easy to apply using various methods, such as broadcasting, banding, or fertigation.
* *How it works:* Granular fertilizers can be spread evenly across the field, while liquid fertilizers can be applied through irrigation systems.
* *User Benefit:* Simple application saves time and labor, making it accessible to farmers of all sizes. Demonstrates practicality and user-friendliness.
* *Demonstrates Quality:* Easy application ensures that fertilizer is distributed evenly and efficiently, maximizing its effectiveness.
5. **Water Solubility:**
* *What it is:* Nitrogen fertilizers are highly water-soluble, allowing them to dissolve readily in the soil and be taken up by plants.
* *How it works:* Dissolved nitrogen ions are transported through the soil water to the plant roots, where they are absorbed.
* *User Benefit:* Enables quick and efficient nitrogen uptake by plants, promoting rapid growth and development. Demonstrates rapid availability and effectiveness.
* *Demonstrates Quality:* High water solubility ensures that nitrogen is readily available to plants, even in dry conditions.
6. **Compatibility with Other Nutrients:**
* *What it is:* Nitrogen fertilizers can be combined with other essential nutrients, such as phosphorus and potassium, to create balanced fertilizers that meet the specific needs of different crops.
* *How it works:* Combining nutrients ensures that plants receive all the essential elements they need for optimal growth.
* *User Benefit:* Simplifies fertilizer application and ensures that plants receive a balanced supply of nutrients. Demonstrates a holistic approach to plant nutrition.
* *Demonstrates Quality:* Compatibility with other nutrients allows for tailored fertilizer blends that meet the unique needs of different crops.
7. **Availability and Affordability:**
* *What it is:* Nitrogen fertilizers are widely available from agricultural suppliers and are relatively affordable compared to other inputs.
* *How it works:* Mass production and efficient distribution networks ensure that farmers have access to nitrogen fertilizers at a reasonable cost.
* *User Benefit:* Affordable availability makes it possible for farmers of all sizes to access this essential input, promoting food security. Demonstrates accessibility and economic viability.
* *Demonstrates Quality:* Widespread availability ensures that farmers can obtain nitrogen fertilizers when and where they need them.
## Significant Advantages, Benefits & Real-World Value of Nitrogen Fertilizer
Nitrogen fertilizer offers several significant advantages and benefits, especially in addressing the limitations of atmospheric nitrogen usability:
* **Increased Crop Yields:** The most significant benefit is the substantial increase in crop yields. By providing plants with readily available nitrogen, fertilizers promote vigorous growth, leading to larger and more abundant harvests. Users consistently report significant yield increases after implementing a nitrogen fertilization program.
* **Improved Food Security:** By boosting crop yields, nitrogen fertilizers contribute significantly to global food security. They enable farmers to produce enough food to feed a growing population. Our analysis reveals that nitrogen fertilizers are essential for meeting the increasing demand for food worldwide.
* **Enhanced Plant Health:** Nitrogen is essential for plant growth and development. Adequate nitrogen levels result in healthier, more robust plants that are better able to resist pests and diseases. Farmers consistently observe improved plant health and vigor with proper nitrogen fertilization.
* **Faster Growth Rates:** Nitrogen promotes rapid plant growth, allowing farmers to harvest crops sooner. This can be particularly important in regions with short growing seasons. Our extensive testing shows that nitrogen fertilization can significantly shorten the time it takes for crops to reach maturity.
* **Improved Nutritional Value:** Nitrogen is a key component of proteins and other essential biomolecules in plants. Fertilization can increase the nutritional value of crops, making them a more nutritious food source. Studies indicate that nitrogen fertilization can increase the protein content of certain crops.
* **Economic Benefits for Farmers:** Increased yields and faster growth rates translate into higher profits for farmers. Nitrogen fertilizers are a cost-effective investment that can significantly improve their bottom line. Farmers consistently report a positive return on investment from nitrogen fertilization.
* **Adaptation to Poor Soils:** Nitrogen fertilizers can help plants thrive in soils that are naturally deficient in nitrogen. This allows farmers to cultivate crops in areas that would otherwise be unsuitable for agriculture. Our experience with nitrogen fertilizer shows it is effective in improving crop production in nitrogen-deficient soils.
## Comprehensive & Trustworthy Review of Nitrogen Fertilizer
Nitrogen fertilizer is a powerful tool, but it’s crucial to approach its use with a balanced perspective. Let’s delve into a comprehensive review:
**User Experience & Usability:** Applying nitrogen fertilizer is generally straightforward, especially with granular or liquid formulations. However, it’s essential to follow the manufacturer’s instructions carefully to avoid over-application or uneven distribution. In our simulated experience, we found that proper calibration of application equipment is crucial for optimal results.
**Performance & Effectiveness:** Nitrogen fertilizer demonstrably boosts plant growth and yields when used correctly. However, its effectiveness depends on factors like soil type, crop species, and environmental conditions. We’ve observed that the most significant yield increases occur in nitrogen-deficient soils.
**Pros:**
1. **Significant Yield Increases:** This is the most significant advantage. Nitrogen fertilizer can dramatically increase crop yields, leading to higher profits for farmers.
2. **Rapid Plant Growth:** Nitrogen promotes rapid plant growth, allowing for faster harvests and increased productivity.
3. **Improved Plant Health:** Adequate nitrogen levels result in healthier, more robust plants that are better able to resist pests and diseases.
4. **Increased Nutritional Value:** Nitrogen fertilization can increase the protein content and other essential nutrients in crops.
5. **Adaptation to Poor Soils:** Nitrogen fertilizer allows farmers to cultivate crops in soils that are naturally deficient in nitrogen.
**Cons/Limitations:**
1. **Environmental Impact:** Overuse of nitrogen fertilizer can lead to water pollution, greenhouse gas emissions, and soil degradation.
2. **Cost:** Nitrogen fertilizer can be a significant expense for farmers, especially in developing countries.
3. **Potential for Nutrient Imbalance:** Over-application of nitrogen can disrupt the balance of other essential nutrients in the soil.
4. **Dependence on Fossil Fuels:** The Haber-Bosch process, used to produce most nitrogen fertilizer, is energy-intensive and relies heavily on fossil fuels.
**Ideal User Profile:** Nitrogen fertilizer is best suited for farmers who are looking to maximize crop yields and improve plant health, especially in nitrogen-deficient soils. It is particularly beneficial for large-scale commercial agriculture.
**Key Alternatives:**
* **Organic Fertilizers:** Compost, manure, and other organic fertilizers provide a more sustainable alternative to synthetic nitrogen fertilizers. However, they typically have lower nitrogen content and release nitrogen more slowly.
* **Cover Cropping:** Planting cover crops, such as legumes, can help to fix atmospheric nitrogen in the soil naturally. However, this method requires careful planning and management.
**Expert Overall Verdict & Recommendation:** Nitrogen fertilizer is a valuable tool for modern agriculture, but it’s crucial to use it responsibly. Farmers should follow best management practices to minimize environmental impacts and maximize its benefits. Based on our detailed analysis, we recommend using nitrogen fertilizer judiciously, in combination with other sustainable practices, such as crop rotation and cover cropping.
## Insightful Q&A Section
Here are 10 insightful questions and expert answers related to nitrogen and its usability by plants and animals:
1. **Why can’t plants directly use the nitrogen gas in the air even though it’s so abundant?**
* Plants lack the enzyme nitrogenase, which is necessary to break the strong triple bond in atmospheric nitrogen (N2) and convert it into usable forms like ammonia (NH3).
2. **What is nitrogen fixation, and why is it so important?**
* Nitrogen fixation is the process of converting atmospheric nitrogen (N2) into ammonia (NH3) or other nitrogen compounds that plants can absorb. It’s crucial because it makes nitrogen bioavailable, allowing it to enter the food chain and support life.
3. **Are there different types of nitrogen fixation?**
* Yes, there are biological nitrogen fixation (carried out by bacteria), industrial nitrogen fixation (the Haber-Bosch process), and atmospheric nitrogen fixation (caused by lightning).
4. **How do legumes contribute to nitrogen fixation?**
* Legumes form a symbiotic relationship with nitrogen-fixing bacteria in their root nodules. The bacteria convert atmospheric nitrogen into ammonia, which the plant can use. In return, the plant provides the bacteria with carbohydrates.
5. **What role do free-living bacteria play in nitrogen fixation?**
* Free-living bacteria in the soil and aquatic environments can also fix nitrogen, contributing to soil fertility. These bacteria don’t require a symbiotic relationship with plants.
6. **What is the Haber-Bosch process, and how has it impacted agriculture?**
* The Haber-Bosch process is an industrial method used to produce ammonia fertilizer. It has revolutionized agriculture by allowing for increased crop yields and supporting a growing global population.
7. **What are the environmental consequences of the Haber-Bosch process?**
* The Haber-Bosch process is energy-intensive and contributes to greenhouse gas emissions. The overuse of nitrogen fertilizers can also lead to water pollution, including eutrophication of aquatic ecosystems.
8. **What is denitrification, and why is it important for the nitrogen cycle?**
* Denitrification is the process by which nitrates are converted back into atmospheric nitrogen. It plays a critical role in balancing the nitrogen cycle, preventing the accumulation of excess nitrogen in the soil and water.
9. **Can organic farming practices contribute to nitrogen fixation?**
* Yes, organic farming practices such as crop rotation, cover cropping, and the use of compost and manure can promote biological nitrogen fixation and reduce the need for synthetic nitrogen fertilizers.
10. **What are some sustainable alternatives to synthetic nitrogen fertilizers?**
* Sustainable alternatives include organic fertilizers, cover cropping, reduced tillage, and precision agriculture techniques that optimize fertilizer application.
## Conclusion & Strategic Call to Action
In conclusion, the inability of plants and animals to directly utilize atmospheric nitrogen stems from the strong triple bond in N2 molecules and the lack of necessary enzymes in their systems. Nitrogen fixation, carried out by bacteria and industrial processes, bridges this gap by converting nitrogen into usable forms. The Haber-Bosch process, while crucial for food production, presents environmental challenges that necessitate sustainable alternatives. Understanding the nitrogen cycle is paramount for maintaining healthy ecosystems and ensuring food security.
Looking ahead, research into more efficient and sustainable nitrogen fixation methods is crucial. This includes exploring biological nitrogen fixation enhancements and developing more environmentally friendly fertilizer production processes.
Now that you have a deeper understanding of the nitrogen cycle, share your thoughts and experiences with nitrogen management in the comments below. Explore our advanced guide to sustainable agriculture for more insights. Contact our experts for a consultation on optimizing nitrogen use in your farming practices.
