## Is Glucose the Only Monomer of a Carbohydrate? A Deep Dive
The question, “Is glucose the only monomer of a carbohydrate?” seems simple on the surface, but delving into the intricacies of biochemistry reveals a more nuanced reality. Many students and even some seasoned professionals stumble on this point. You likely searched this because you’re trying to understand the building blocks of life, specifically how carbohydrates are constructed. This comprehensive guide will not only definitively answer this question but will also explore the diverse world of monosaccharides and their roles in forming complex carbohydrates, providing clarity and a solid foundation for understanding carbohydrate chemistry. We aim to provide a resource far exceeding typical online explanations in depth and clarity.
### What You’ll Learn
* A clear understanding of the definition of a monomer and a carbohydrate.
* Identification of various monosaccharides beyond glucose.
* Explanation of how different monosaccharides combine to form disaccharides, oligosaccharides, and polysaccharides.
* Insight into the biological significance of diverse carbohydrate structures.
* Debunking common misconceptions about carbohydrate composition.
## Deep Dive: Decoding Monomers and Carbohydrates
To tackle the question of whether glucose is the only monomer of a carbohydrate, we must first establish a firm understanding of the fundamental concepts involved: monomers and carbohydrates themselves.
### Monomers: The Building Blocks of Polymers
A monomer is a small molecule that can bind chemically to other molecules of the same type to form a polymer. Think of monomers as individual Lego bricks; they are the basic units that can be linked together to create larger, more complex structures. In the realm of biological macromolecules, monomers are the fundamental building blocks of proteins (amino acids), nucleic acids (nucleotides), and, most importantly for our discussion, carbohydrates (monosaccharides).
### Carbohydrates: Fuel, Structure, and Information
Carbohydrates, also known as saccharides, are organic compounds composed of carbon, hydrogen, and oxygen, typically in a 1:2:1 ratio (CH2O)n. They serve a multitude of crucial functions in living organisms, including:
* **Energy Source:** Carbohydrates are the primary source of energy for most living organisms. Glucose, in particular, is readily metabolized to produce ATP, the cell’s energy currency.
* **Energy Storage:** Carbohydrates like starch (in plants) and glycogen (in animals) act as energy reserves, providing a readily available source of glucose when needed.
* **Structural Components:** Carbohydrates form essential structural components in cell walls (cellulose in plants, peptidoglycan in bacteria) and exoskeletons (chitin in arthropods).
* **Cellular Communication:** Carbohydrates attached to proteins and lipids on the cell surface (glycoproteins and glycolipids) play vital roles in cell recognition, signaling, and immune responses.
### The Hierarchy of Carbohydrates
Carbohydrates are classified based on the number of sugar units they contain:
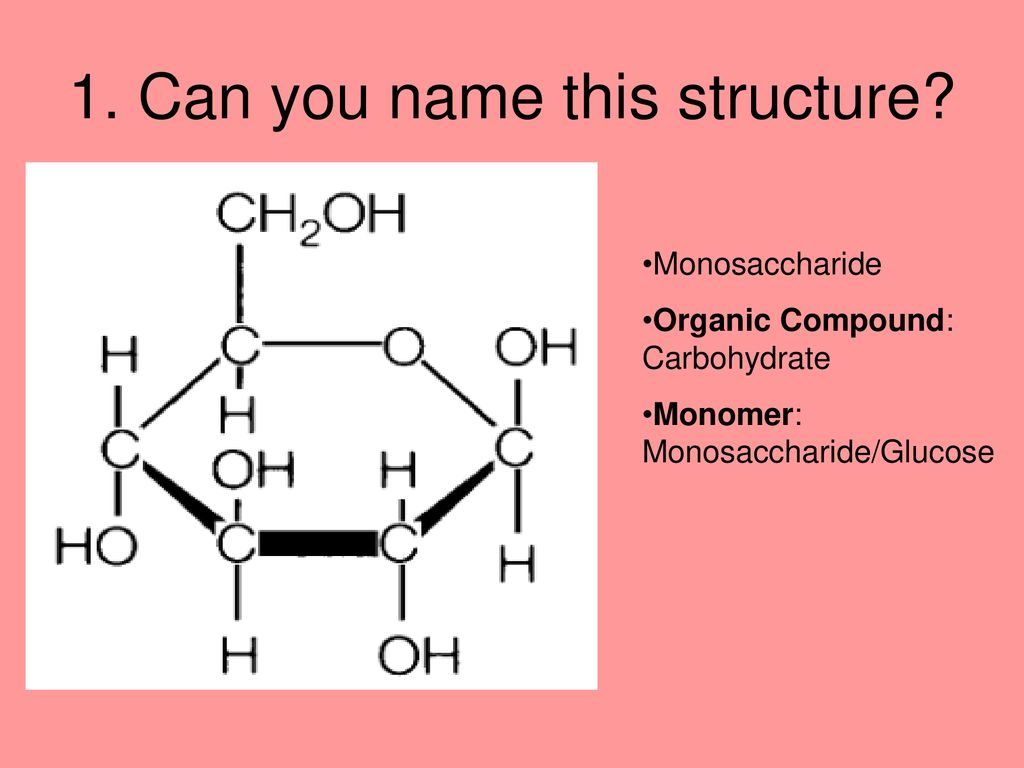
* **Monosaccharides:** These are the simplest sugars, consisting of a single sugar unit. Examples include glucose, fructose, and galactose. These are, by definition, monomers of carbohydrates.
* **Disaccharides:** These are formed when two monosaccharides are joined together by a glycosidic bond. Examples include sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (glucose + glucose).
* **Oligosaccharides:** These consist of a small number (typically 3-10) of monosaccharides linked together. They are often found attached to proteins and lipids on cell surfaces and play roles in cell recognition and signaling.
* **Polysaccharides:** These are complex carbohydrates consisting of many (hundreds or thousands) monosaccharides linked together. Examples include starch, glycogen, cellulose, and chitin.
### The Truth: Glucose is *Not* the Only Monomer
Now, let’s directly address the core question: Is glucose the only monomer of a carbohydrate? The answer is definitively **no**. While glucose is arguably the most important and abundant monosaccharide, it is not the only one. Other monosaccharides, such as fructose, galactose, ribose, and deoxyribose, also serve as monomers in various carbohydrates. These diverse monosaccharides contribute to the structural and functional diversity of carbohydrates.
* **Fructose:** Found in fruits and honey, fructose is a key component of sucrose (table sugar).
* **Galactose:** Found in milk, galactose combines with glucose to form lactose.
* **Ribose:** A five-carbon sugar that is a component of RNA.
* **Deoxyribose:** A modified form of ribose that is a component of DNA.
The different properties of these monosaccharides, like their sweetness and how they are metabolized, contribute to the unique characteristics of the larger carbohydrates they form.
## Key Monosaccharides Beyond Glucose: An Expert’s Overview
While glucose takes center stage in many metabolic processes, other monosaccharides play equally vital roles. Understanding these monomers provides a more comprehensive picture of carbohydrate biochemistry.
### Fructose: The Fruit Sugar
Fructose, also known as fruit sugar, is a ketose monosaccharide (meaning it has a ketone group). It is significantly sweeter than glucose and is abundant in fruits, honey, and high-fructose corn syrup. Fructose is metabolized differently than glucose, primarily in the liver. Excessive fructose consumption has been linked to various health issues, highlighting the importance of understanding its metabolic pathway.
### Galactose: The Milk Sugar Component
Galactose is an aldose monosaccharide (meaning it has an aldehyde group) and is a component of lactose, the sugar found in milk. Galactose is converted to glucose in the liver for energy production. Individuals with lactose intolerance have difficulty digesting lactose because they lack the enzyme lactase, which is required to break the bond between glucose and galactose.
### Ribose and Deoxyribose: The Nucleic Acid Sugars
Ribose and deoxyribose are five-carbon sugars (pentoses) that are essential components of RNA and DNA, respectively. Ribose contains a hydroxyl group (-OH) on the 2′ carbon, while deoxyribose lacks this oxygen atom (hence the name “deoxy”). These sugars form the backbone of nucleic acids, providing the structural framework for genetic information.
### Other Important Monosaccharides
Beyond these common examples, other monosaccharides play specialized roles in biological systems:
* **Mannose:** Found in glycoproteins and polysaccharides in plants and bacteria.
* **Xylose:** A component of hemicellulose in plant cell walls.
* **Arabinose:** Found in plant gums and pectins.
## Disaccharides and Polysaccharides: Building Complexity
Monosaccharides are the foundation, but the real magic happens when they combine to form more complex carbohydrates. This section explores how different monosaccharides link together to create disaccharides and polysaccharides with diverse properties and functions.
### Disaccharides: Two Sugars Joined Together
Disaccharides are formed when two monosaccharides are joined by a glycosidic bond, a covalent bond formed through dehydration (removal of water). Common disaccharides include:
* **Sucrose (Table Sugar):** Glucose + Fructose. Sucrose is the primary form of sugar transported in plants and is widely used as a sweetener.
* **Lactose (Milk Sugar):** Glucose + Galactose. Lactose is the sugar found in milk and is a crucial source of energy for infant mammals.
* **Maltose (Malt Sugar):** Glucose + Glucose. Maltose is produced during the germination of grains and is an intermediate product in starch digestion.
The specific properties of each disaccharide depend on the monosaccharides involved and the type of glycosidic bond that links them.
### Polysaccharides: Long Chains of Sugars
Polysaccharides are complex carbohydrates consisting of long chains of monosaccharides linked together by glycosidic bonds. They can be linear or branched and serve diverse functions, including energy storage and structural support. Key examples include:
* **Starch:** A storage polysaccharide in plants, composed of glucose monomers in the form of amylose (linear) and amylopectin (branched). Starch is a major source of energy for humans and animals.
* **Glycogen:** A storage polysaccharide in animals, similar to amylopectin but more highly branched. Glycogen is stored in the liver and muscles and provides a readily available source of glucose.
* **Cellulose:** A structural polysaccharide in plant cell walls, composed of glucose monomers linked by β-1,4-glycosidic bonds. Cellulose is the most abundant organic compound on Earth and provides structural support to plants.
* **Chitin:** A structural polysaccharide in the exoskeletons of arthropods and cell walls of fungi, composed of N-acetylglucosamine monomers. Chitin provides strength and rigidity to these structures.
The diversity of polysaccharides arises from the different monosaccharides involved, the type of glycosidic bonds, and the degree of branching. These variations dictate the physical and chemical properties of the polysaccharides and their specific biological functions.
## Why This Matters: Biological Significance and Real-World Applications
Understanding the diverse roles of monosaccharides and their polymers is crucial for comprehending fundamental biological processes and addressing real-world challenges in nutrition, medicine, and biotechnology.
### Nutritional Implications
The types of carbohydrates we consume have a profound impact on our health. Simple sugars like glucose and fructose provide quick energy but can lead to rapid spikes in blood sugar levels. Complex carbohydrates like starch and cellulose are digested more slowly, providing a sustained release of energy and promoting better blood sugar control. Dietary fiber, composed of non-digestible polysaccharides like cellulose, is essential for digestive health and overall well-being. A balanced diet that includes a variety of carbohydrates from whole grains, fruits, and vegetables is crucial for optimal health.
### Medical Applications
Carbohydrates play vital roles in various medical applications:
* **Drug Delivery:** Polysaccharides are used to encapsulate and deliver drugs to specific targets in the body.
* **Tissue Engineering:** Carbohydrate-based materials are used as scaffolds for tissue regeneration.
* **Diagnostics:** Carbohydrate antigens are used to detect and diagnose various diseases.
### Biotechnological Applications
Carbohydrates are also widely used in biotechnology:
* **Biofuels:** Carbohydrates from biomass are converted into biofuels like ethanol.
* **Bioplastics:** Carbohydrates are used to produce biodegradable plastics.
* **Food Industry:** Carbohydrates are used as thickeners, stabilizers, and sweeteners in food products.
## Common Misconceptions About Carbohydrate Composition
It’s easy to fall into traps of misinformation when learning about complex topics. Let’s debunk some common misconceptions surrounding carbohydrate composition:
* **Misconception:** All carbohydrates are bad for you.
* **Reality:** Not all carbohydrates are created equal. While excessive consumption of refined sugars can be detrimental to health, complex carbohydrates from whole grains, fruits, and vegetables are essential for energy, fiber, and overall well-being.
* **Misconception:** Glucose is the only sugar the body can use.
* **Reality:** While glucose is a primary fuel source, the body can convert other monosaccharides, like fructose and galactose, into glucose for energy production.
* **Misconception:** Starch is just a simple chain of glucose molecules.
* **Reality:** Starch is a complex mixture of amylose (linear chains of glucose) and amylopectin (branched chains of glucose), each with distinct properties.
## Q&A: Addressing Your Burning Questions About Carbohydrates
Here are some frequently asked questions about carbohydrates, along with expert answers:
1. **What is the difference between simple and complex carbohydrates?**
* Simple carbohydrates (monosaccharides and disaccharides) are quickly digested and provide a rapid source of energy, while complex carbohydrates (oligosaccharides and polysaccharides) are digested more slowly, providing a sustained release of energy.
2. **How does the body digest carbohydrates?**
* Carbohydrate digestion begins in the mouth with salivary amylase, which breaks down starch into smaller oligosaccharides. Further digestion occurs in the small intestine with pancreatic amylase and enzymes that break down disaccharides into monosaccharides, which are then absorbed into the bloodstream.
3. **What is the glycemic index (GI)?**
* The glycemic index (GI) is a measure of how quickly a food raises blood sugar levels compared to glucose. Foods with a high GI cause rapid spikes in blood sugar, while foods with a low GI cause a more gradual rise.
4. **What are the benefits of eating fiber?**
* Fiber promotes digestive health by adding bulk to the stool, preventing constipation, and promoting the growth of beneficial gut bacteria. It can also help lower cholesterol levels and regulate blood sugar.
5. **Are artificial sweeteners carbohydrates?**
* No, artificial sweeteners are not carbohydrates. They are synthetic compounds that provide sweetness without contributing significant calories or affecting blood sugar levels. However, some sugar alcohols (like xylitol and sorbitol) are considered carbohydrates.
6. **How do different types of glycosidic bonds affect polysaccharide structure?**
* The type of glycosidic bond (alpha or beta) determines the three-dimensional structure of the polysaccharide. For example, the beta-1,4-glycosidic bonds in cellulose create a linear, rigid structure that provides structural support to plant cell walls, while the alpha-1,4-glycosidic bonds in starch create a more flexible structure that allows for energy storage.
7. **What role do carbohydrates play in cell signaling?**
* Carbohydrates attached to proteins and lipids on the cell surface (glycoproteins and glycolipids) play crucial roles in cell recognition, signaling, and immune responses. These carbohydrates can act as ligands for receptors on other cells, triggering specific signaling pathways.
8. **How does the body store excess glucose?**
* The body stores excess glucose as glycogen in the liver and muscles. When blood sugar levels are high, insulin stimulates the conversion of glucose to glycogen. When blood sugar levels are low, glycogen is broken down into glucose to maintain energy balance.
9. **What is the difference between starch and cellulose?**
* Both starch and cellulose are composed of glucose monomers, but they differ in the type of glycosidic bond that links the glucose units. Starch has alpha-1,4-glycosidic bonds, while cellulose has beta-1,4-glycosidic bonds. This difference in bonding results in different structures and properties. Starch is digestible by humans, while cellulose is not.
10. **Why is high-fructose corn syrup (HFCS) controversial?**
* HFCS is controversial because it is metabolized differently than glucose. Fructose is primarily metabolized in the liver, and excessive consumption of HFCS has been linked to various health issues, including fatty liver disease, insulin resistance, and obesity.
## Conclusion: Embracing Carbohydrate Diversity
In conclusion, while glucose is a vital and prevalent monosaccharide, it is definitively **not** the only monomer of a carbohydrate. Fructose, galactose, ribose, deoxyribose, and other monosaccharides each contribute unique properties and functions to the diverse world of carbohydrates. Understanding this diversity is essential for comprehending the fundamental roles carbohydrates play in biology, nutrition, medicine, and biotechnology. By moving beyond the simplistic notion of glucose as the sole building block, we gain a richer and more accurate understanding of these essential biomolecules.
Consider exploring advanced texts on biochemistry to further your understanding. Sharing this article with others who are learning about carbohydrates can help spread accurate knowledge and dispel common misconceptions.
## Strategic Call to Action
Share your insights or questions about the different types of monosaccharides in the comments below! We encourage you to explore our other resources on related biochemical topics for a deeper understanding. Contact us if you have specific research needs related to carbohydrate chemistry.
