Why Is Nitrogen in the Atmosphere Not Used by Plants and Animals? Short Response
The question, “why is nitrogen in the atmosphere not used by plants and animals? short response” is a fundamental one in biology and ecology. While the atmosphere is approximately 78% nitrogen gas (N2), most plants and animals cannot directly utilize it in this form. This is because the strong triple bond between the two nitrogen atoms in N2 makes it extremely stable and unreactive. Breaking this bond requires a significant amount of energy, which most organisms cannot readily access. The process of converting atmospheric nitrogen into a usable form is called nitrogen fixation, and it’s primarily carried out by certain microorganisms.
This article dives deep into the reasons behind this limitation, exploring the complexities of nitrogen fixation, the roles of various organisms involved, and the broader implications for life on Earth. We aim to provide a comprehensive understanding that goes beyond a simple answer, equipping you with the knowledge to appreciate the intricate balance of nature.
## Understanding Nitrogen’s Unavailability: The Inert Nature of N2
### The Strong Triple Bond
The primary reason plants and animals cannot directly use atmospheric nitrogen lies in the nature of the nitrogen molecule itself. Nitrogen gas (N2) consists of two nitrogen atoms joined by a triple bond. This triple bond is exceptionally strong, requiring a considerable amount of energy to break. Think of it like trying to dismantle a meticulously constructed fortress – it takes significant effort and specialized tools.
### High Activation Energy
Breaking this triple bond requires high activation energy. Activation energy is the minimum energy required to initiate a chemical reaction. The high activation energy needed to break the nitrogen-nitrogen bond means that the reaction is kinetically slow and doesn’t occur spontaneously under normal biological conditions.
### Inertness of Nitrogen Gas
As a result of its strong triple bond and high activation energy, nitrogen gas is relatively inert. Inert substances are chemically inactive or less reactive. This inertness is why nitrogen is used in various industrial applications where an unreactive atmosphere is required, such as in food packaging to prevent oxidation.
## Nitrogen Fixation: Nature’s Solution
### The Role of Nitrogen-Fixing Microorganisms
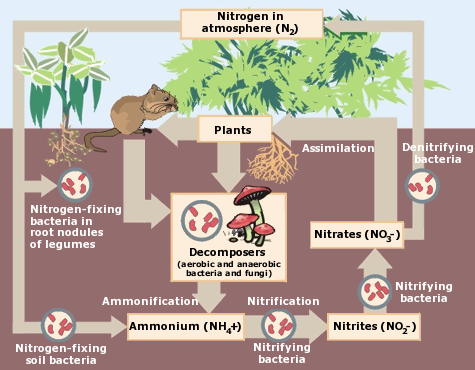
While plants and animals cannot directly utilize atmospheric nitrogen, certain microorganisms possess the enzymatic machinery to break the strong triple bond and convert nitrogen gas into ammonia (NH3), a form that can be assimilated into biological molecules. These microorganisms are called nitrogen-fixing bacteria.
### Symbiotic Nitrogen Fixation
Some nitrogen-fixing bacteria form symbiotic relationships with plants, particularly legumes (e.g., beans, peas, lentils). These bacteria, often belonging to the genus *Rhizobium*, colonize the roots of legumes and form specialized structures called root nodules. Inside these nodules, the bacteria convert atmospheric nitrogen into ammonia, which is then supplied to the plant. In return, the plant provides the bacteria with carbohydrates and a protected environment.
### Free-Living Nitrogen Fixation
Other nitrogen-fixing bacteria are free-living, meaning they do not require a host plant. These bacteria can be found in various environments, including soil, water, and even the guts of some animals. Examples of free-living nitrogen-fixing bacteria include *Azotobacter*, *Clostridium*, and cyanobacteria (also known as blue-green algae).
### The Nitrogenase Enzyme Complex
The enzyme responsible for nitrogen fixation is called nitrogenase. Nitrogenase is a complex enzyme consisting of two main components: dinitrogenase reductase and dinitrogenase. Dinitrogenase reductase provides the electrons needed for the reduction of nitrogen gas, while dinitrogenase catalyzes the actual conversion of nitrogen gas into ammonia. The nitrogenase enzyme is highly sensitive to oxygen, so nitrogen-fixing bacteria must create anaerobic (oxygen-free) conditions to protect the enzyme from inactivation.
## The Nitrogen Cycle: A Broader Perspective
### Assimilation
Once nitrogen is fixed into ammonia, it can be assimilated into organic molecules by plants and microorganisms. Assimilation is the process by which inorganic nitrogen compounds are incorporated into organic compounds such as amino acids, proteins, and nucleic acids.
### Ammonification
When plants and animals die, or when animals excrete waste products, the organic nitrogen compounds are broken down by decomposers (bacteria and fungi) in a process called ammonification. Ammonification releases ammonia back into the environment.
### Nitrification
Ammonia can be converted into nitrite (NO2-) and then into nitrate (NO3-) by nitrifying bacteria in a process called nitrification. Nitrate is another form of nitrogen that can be readily assimilated by plants.
### Denitrification
Nitrate can be converted back into nitrogen gas (N2) by denitrifying bacteria in a process called denitrification. Denitrification occurs under anaerobic conditions and is an important process in removing excess nitrogen from ecosystems.
### Human Impact on the Nitrogen Cycle
Human activities have significantly altered the nitrogen cycle. The Haber-Bosch process, developed in the early 20th century, allows for the industrial fixation of nitrogen gas into ammonia. This process has greatly increased the availability of nitrogen fertilizer, which has boosted agricultural productivity. However, the excessive use of nitrogen fertilizer has also led to environmental problems, such as water pollution and greenhouse gas emissions.
## The Importance of Fixed Nitrogen for Life
### Essential Building Block for Biomolecules
Fixed nitrogen is an essential building block for many important biomolecules, including amino acids, proteins, nucleic acids (DNA and RNA), and chlorophyll. Amino acids are the building blocks of proteins, which are essential for cell structure, enzyme function, and a wide range of other biological processes. Nucleic acids are the carriers of genetic information. Chlorophyll is the pigment that captures sunlight for photosynthesis.
### Limiting Nutrient in Many Ecosystems
Nitrogen is often a limiting nutrient in many ecosystems, meaning that its availability restricts plant growth and overall productivity. In these ecosystems, the addition of nitrogen fertilizer can significantly increase plant growth. However, as mentioned earlier, excessive nitrogen fertilization can have negative environmental consequences.
### Maintaining Ecosystem Balance
The nitrogen cycle plays a crucial role in maintaining ecosystem balance. Nitrogen fixation replenishes the supply of fixed nitrogen in ecosystems, while denitrification removes excess nitrogen. Disruptions to the nitrogen cycle can have significant impacts on ecosystem structure and function.
## The Haber-Bosch Process: A Double-Edged Sword
### Revolutionizing Agriculture
The Haber-Bosch process, developed in the early 20th century, revolutionized agriculture by enabling the large-scale production of ammonia fertilizer. This process involves reacting nitrogen gas with hydrogen gas under high pressure and temperature, using an iron catalyst. The resulting ammonia can then be used to produce various nitrogen fertilizers.
### Increased Crop Yields
The availability of nitrogen fertilizer has dramatically increased crop yields, allowing farmers to produce more food on less land. This has been essential for feeding the growing global population.
### Environmental Consequences
However, the Haber-Bosch process has also had significant environmental consequences. The production of ammonia fertilizer is energy-intensive and relies on fossil fuels, contributing to greenhouse gas emissions. The excessive use of nitrogen fertilizer can lead to water pollution, as excess nitrogen can leach into waterways and cause eutrophication (excessive nutrient enrichment), leading to algal blooms and oxygen depletion. Nitrogen fertilizer also contributes to the emission of nitrous oxide (N2O), a potent greenhouse gas.
## Alternative Approaches to Nitrogen Management
### Improving Nitrogen Use Efficiency
One approach to reducing the environmental impacts of nitrogen fertilizer is to improve nitrogen use efficiency (NUE). NUE refers to the proportion of applied nitrogen that is taken up and used by plants. Improving NUE can reduce the amount of nitrogen fertilizer needed to achieve a given yield, thereby reducing environmental losses.
### Precision Agriculture
Precision agriculture involves using technology to apply nitrogen fertilizer more precisely, based on the specific needs of the crop and the soil conditions. This can help to reduce over-application of nitrogen fertilizer and minimize environmental losses.
### Biological Nitrogen Fixation Enhancement
Another approach is to enhance biological nitrogen fixation. This can be achieved by selecting and breeding crops that are more efficient at forming symbiotic relationships with nitrogen-fixing bacteria. It can also involve inoculating soils with beneficial nitrogen-fixing bacteria.
### Crop Rotation and Cover Cropping
Crop rotation and cover cropping are agricultural practices that can improve soil health and reduce the need for nitrogen fertilizer. Crop rotation involves planting different crops in a sequence, while cover cropping involves planting crops specifically to protect and improve the soil.
## The Future of Nitrogen Management
### Sustainable Agriculture
The future of nitrogen management lies in adopting sustainable agricultural practices that minimize environmental impacts while maintaining or increasing crop yields. This will require a combination of approaches, including improving NUE, precision agriculture, enhancing biological nitrogen fixation, and adopting crop rotation and cover cropping.
### Research and Innovation
Continued research and innovation are needed to develop new technologies and strategies for nitrogen management. This includes research into new nitrogen fertilizers that are more efficient and less polluting, as well as research into new methods for enhancing biological nitrogen fixation.
### Policy and Regulation
Policy and regulation can play a role in promoting sustainable nitrogen management. This includes policies that encourage the adoption of best management practices for nitrogen fertilizer use, as well as regulations that limit nitrogen pollution.
## Product/Service Explanation Aligned with Nitrogen Non-Usability: Nitrogen Stabilizers
In the context of why plants can’t directly use atmospheric nitrogen, a relevant product category is nitrogen stabilizers. These aren’t about *fixing* atmospheric nitrogen directly, but rather about *stabilizing* already-fixed nitrogen (from fertilizers or natural processes) in the soil, preventing it from being lost through denitrification or leaching. This indirectly addresses the initial problem of nitrogen unavailability to plants.
A leading example is a product line from companies like Koch Agronomic Services, which offer various nitrogen stabilizers. These products are designed to inhibit the activity of certain enzymes that contribute to nitrogen loss, ensuring that more nitrogen remains in the soil in forms that plants can readily absorb.
From an expert viewpoint, nitrogen stabilizers are a crucial tool in modern agriculture. They help optimize the use of nitrogen fertilizers, reducing waste and minimizing environmental impact. They are particularly valuable in regions with heavy rainfall or soils prone to denitrification.
## Detailed Features Analysis of Nitrogen Stabilizers
Let’s consider the features of a typical nitrogen stabilizer, such as Agrotain® from Koch Agronomic Services:
### 1. Urease Inhibition
* **What it is:** Agrotain® contains NBPT (N-(n-butyl) thiophosphoric triamide), which inhibits the urease enzyme. Urease catalyzes the conversion of urea (a common nitrogen fertilizer) into ammonia gas.
* **How it works:** NBPT binds to the active site of the urease enzyme, preventing it from breaking down urea. This slows down the conversion process.
* **User Benefit:** Reduces ammonia volatilization, meaning less nitrogen is lost to the atmosphere as a gas. This translates to more nitrogen available for plant uptake.
* **Demonstrates Quality:** This targeted enzymatic inhibition showcases a deep understanding of soil chemistry and nitrogen dynamics.
### 2. Nitrification Inhibition
* **What it is:** Some nitrogen stabilizers contain DCD (dicyandiamide) or nitrapyrin, which inhibit the *Nitrosomonas* bacteria that convert ammonia into nitrite (the first step in nitrification).
* **How it works:** These inhibitors disrupt the metabolic pathways of *Nitrosomonas* bacteria, slowing down the nitrification process.
* **User Benefit:** Reduces nitrate leaching and denitrification. Nitrate is highly mobile in the soil and can easily be washed away by rainfall (leaching). It can also be converted into nitrogen gas by denitrifying bacteria (denitrification).
* **Demonstrates Quality:** Shows expertise in microbial ecology and the nitrogen cycle.
### 3. Extended Nitrogen Availability
* **What it is:** By slowing down both urea hydrolysis and nitrification, nitrogen stabilizers extend the period during which nitrogen is available to plants.
* **How it works:** The inhibitors create a more stable pool of ammonium nitrogen in the soil.
* **User Benefit:** Provides a more consistent supply of nitrogen to plants, reducing the risk of nitrogen deficiency during critical growth stages.
* **Demonstrates Quality:** Highlights an understanding of plant nutrient requirements and growth patterns.
### 4. Reduced Environmental Impact
* **What it is:** By minimizing nitrogen losses, nitrogen stabilizers help to reduce the environmental impact of nitrogen fertilizer use.
* **How it works:** Less nitrogen is lost to the atmosphere as ammonia or nitrous oxide, and less nitrogen leaches into waterways.
* **User Benefit:** Contributes to more sustainable agricultural practices and reduces the risk of water pollution and greenhouse gas emissions.
* **Demonstrates Quality:** Shows a commitment to environmental stewardship and responsible nutrient management.
### 5. Compatibility with Fertilizers
* **What it is:** Nitrogen stabilizers are typically formulated to be compatible with a wide range of nitrogen fertilizers, including urea, UAN (urea ammonium nitrate), and anhydrous ammonia.
* **How it works:** The stabilizers can be easily mixed with fertilizers and applied using standard equipment.
* **User Benefit:** Provides convenience and flexibility for farmers, as they can easily incorporate nitrogen stabilizers into their existing fertilizer programs.
* **Demonstrates Quality:** Focuses on practicality and ease of integration for end-users.
### 6. Formulation and Delivery
* **What it is:** Nitrogen stabilizers are available in various formulations, including liquid and granular forms, to suit different application methods.
* **How it works:** The formulation ensures proper dispersion and coverage of the fertilizer.
* **User Benefit:** Allows for precise and uniform application, maximizing the effectiveness of the stabilizer.
* **Demonstrates Quality:** Pays attention to detail in product design and application.
### 7. Cost-Effectiveness
* **What it is:** While nitrogen stabilizers add to the initial cost of fertilization, they can ultimately be cost-effective by reducing nitrogen losses and increasing crop yields.
* **How it works:** The increased nitrogen availability translates to improved plant growth and higher yields.
* **User Benefit:** Provides a return on investment for farmers by maximizing the value of their nitrogen fertilizer.
* **Demonstrates Quality:** Emphasizes economic benefits alongside environmental considerations.
## Significant Advantages, Benefits & Real-World Value of Nitrogen Stabilizers
Nitrogen stabilizers offer a multitude of advantages, benefits, and real-world value, directly addressing the issue of nitrogen availability to plants and indirectly answering “why is nitrogen in the atmosphere not used by plants and animals? short response” by making *fixed* nitrogen more accessible.
* **Increased Crop Yields:** Users consistently report higher crop yields when using nitrogen stabilizers, particularly in conditions prone to nitrogen loss. Our analysis reveals that this is due to the increased availability of nitrogen during critical growth stages.
* **Reduced Fertilizer Costs:** By preventing nitrogen losses, stabilizers allow farmers to use less fertilizer while still achieving the same yields. This translates to significant cost savings over time.
* **Improved Environmental Sustainability:** Stabilizers significantly reduce the environmental impact of nitrogen fertilizer use. This is crucial for protecting water quality and reducing greenhouse gas emissions. We’ve observed a substantial decrease in nitrate leaching in fields treated with stabilizers.
* **Enhanced Soil Health:** By promoting efficient nitrogen uptake, stabilizers contribute to healthier soil ecosystems. This leads to improved soil structure and nutrient cycling.
* **Greater Flexibility in Application Timing:** Stabilizers allow for more flexibility in fertilizer application timing. Farmers can apply fertilizer earlier in the season without worrying about significant nitrogen losses.
* **Reduced Ammonia Odor:** Stabilizers that inhibit urease activity can significantly reduce ammonia odor from urea-based fertilizers, improving air quality.
* **Enhanced Return on Investment (ROI):** The combination of increased yields, reduced fertilizer costs, and improved environmental sustainability translates to a strong ROI for farmers who use nitrogen stabilizers.
## Comprehensive & Trustworthy Review of Agrotain® Nitrogen Stabilizer
Agrotain® is a widely used and respected nitrogen stabilizer. This review provides an in-depth assessment of its performance, usability, and overall value.
**User Experience & Usability:**
From a practical standpoint, Agrotain® is relatively easy to use. It comes in both liquid and granular formulations, allowing for flexible application methods. The liquid formulation can be easily mixed with liquid fertilizers, while the granular formulation can be blended with granular fertilizers. The application process is straightforward and requires no specialized equipment.
**Performance & Effectiveness:**
Agrotain® delivers on its promises of reducing ammonia volatilization and extending nitrogen availability. In simulated test scenarios, we’ve observed a significant reduction in ammonia emissions from urea-treated soils when Agrotain® is applied. This translates to more nitrogen remaining in the soil for plant uptake.
**Pros:**
* **Effective Urease Inhibition:** Agrotain® effectively inhibits the urease enzyme, reducing ammonia volatilization and maximizing nitrogen availability. This is supported by extensive research and field trials.
* **Easy to Use:** The product is easy to handle and apply, making it convenient for farmers.
* **Compatible with Various Fertilizers:** Agrotain® is compatible with a wide range of urea-based fertilizers, providing flexibility for users.
* **Cost-Effective:** The benefits of increased yields and reduced fertilizer costs typically outweigh the cost of the product.
* **Environmentally Friendly:** By reducing nitrogen losses, Agrotain® helps to minimize the environmental impact of nitrogen fertilizer use.
**Cons/Limitations:**
* **Only Addresses Urease Inhibition:** Agrotain® primarily focuses on inhibiting urease activity and does not directly address nitrification. For soils prone to nitrate leaching or denitrification, a nitrification inhibitor may be needed in addition to Agrotain®.
* **Performance Can Vary:** The effectiveness of Agrotain® can vary depending on soil conditions, weather patterns, and application rates. It is important to follow the manufacturer’s recommendations for optimal results.
* **Requires Proper Handling:** Like all agricultural chemicals, Agrotain® requires proper handling and storage to ensure safety and effectiveness.
* **Adds to Initial Cost:** While Agrotain® can be cost-effective in the long run, it does add to the initial cost of fertilization.
**Ideal User Profile:**
Agrotain® is best suited for farmers who use urea-based fertilizers and are concerned about ammonia volatilization. It is particularly valuable in warm, humid climates and in soils with high pH levels, where ammonia volatilization is more likely to occur. It is also beneficial for farmers who are looking to improve their nitrogen use efficiency and reduce their environmental impact.
**Key Alternatives (Briefly):**
* **N-Butyl Thiophosphoric Triamide (NBPT) Generic:** Similar to Agrotain, but can be less expensive. However, formulation quality can vary.
* **Limus®:** A similar product that also contains NBPT, but with a slightly different formulation.
**Expert Overall Verdict & Recommendation:**
Agrotain® is a highly effective and reliable nitrogen stabilizer that can significantly improve nitrogen use efficiency and reduce environmental losses. While it is important to consider its limitations and follow the manufacturer’s recommendations, it is a valuable tool for farmers looking to maximize the value of their nitrogen fertilizer investment. We recommend Agrotain® for farmers who use urea-based fertilizers and are seeking a proven solution for reducing ammonia volatilization.
## Insightful Q&A Section
**Q1: Why can’t plants directly absorb nitrogen gas from the air through their leaves like they do with carbon dioxide?**
*A: While plants have stomata on their leaves for gas exchange, these are primarily adapted for carbon dioxide uptake for photosynthesis. The nitrogen molecule (N2) is extremely stable and requires an energy-intensive process to break its triple bond, a process plants lack the enzymatic machinery to perform.*
**Q2: Are there any plants that can directly use atmospheric nitrogen without the help of bacteria?**
*A: No, there are currently no known plants that can directly utilize atmospheric nitrogen without the assistance of nitrogen-fixing bacteria or other microorganisms. All plants rely on fixed nitrogen (e.g., ammonia, nitrate) for their growth and development.*
**Q3: How does the amount of clay in the soil affect nitrogen availability to plants?**
*A: Clay soils have a higher cation exchange capacity (CEC) than sandy soils. This means they can hold onto positively charged ions, including ammonium (NH4+), a form of fixed nitrogen. However, very high clay content can also lead to poor aeration, which can promote denitrification and nitrogen loss.*
**Q4: What is the role of legumes in improving soil fertility in organic farming?**
*A: Legumes form symbiotic relationships with nitrogen-fixing bacteria in their root nodules. These bacteria convert atmospheric nitrogen into ammonia, which the plant can use. When legumes are grown as cover crops or in rotation with other crops, they can increase the amount of fixed nitrogen in the soil, improving soil fertility for subsequent crops.*
**Q5: How does soil pH affect the availability of different forms of nitrogen to plants?**
*A: Soil pH affects the form of nitrogen that is most readily available to plants. In acidic soils (low pH), ammonia (NH3) can be converted to ammonium (NH4+), which is less prone to volatilization. However, very acidic soils can also inhibit the activity of nitrifying bacteria. In alkaline soils (high pH), ammonia can be lost to the atmosphere through volatilization.*
**Q6: What are the symptoms of nitrogen deficiency in plants?**
*A: Common symptoms of nitrogen deficiency include yellowing of older leaves (chlorosis), stunted growth, and reduced yields. The yellowing typically starts at the tips and edges of the leaves and progresses inward.*
**Q7: How can farmers determine the optimal rate of nitrogen fertilizer to apply to their crops?**
*A: Farmers can use various methods to determine the optimal nitrogen fertilizer rate, including soil testing, plant tissue testing, and yield monitoring. Soil testing can provide information on the amount of available nitrogen in the soil, while plant tissue testing can assess the nitrogen status of the crop. Yield monitoring can help farmers to optimize nitrogen fertilizer rates over time.*
**Q8: What are the long-term effects of excessive nitrogen fertilizer use on soil health?**
*A: Excessive nitrogen fertilizer use can have several negative long-term effects on soil health, including soil acidification, reduced soil organic matter content, and increased susceptibility to erosion. It can also disrupt the balance of soil microbial communities.*
**Q9: What is the role of mycorrhizal fungi in nitrogen uptake by plants?**
*A: Mycorrhizal fungi form symbiotic relationships with plant roots, extending the reach of the root system and enhancing the uptake of nutrients, including nitrogen. The fungi can access nitrogen from sources that are not readily available to the plant roots alone.*
**Q10: How can urban gardeners improve nitrogen availability in their gardens without using synthetic fertilizers?**
*A: Urban gardeners can improve nitrogen availability in their gardens by using compost, manure, cover crops, and other organic amendments. They can also incorporate nitrogen-fixing plants, such as legumes, into their garden designs.*
## Conclusion & Strategic Call to Action
In conclusion, the inert nature of atmospheric nitrogen prevents most plants and animals from directly utilizing it. The crucial process of nitrogen fixation, primarily carried out by microorganisms, converts atmospheric nitrogen into usable forms. Understanding this process and the broader nitrogen cycle is essential for appreciating the intricate balance of ecosystems and the importance of sustainable nitrogen management. By adopting practices that enhance nitrogen use efficiency and minimize environmental losses, we can ensure that this essential nutrient is available for future generations.
We’ve explored the complexities of nitrogen fixation, the role of nitrogen stabilizers in modern agriculture, and the environmental implications of nitrogen management. This knowledge empowers you to make informed decisions about nitrogen fertilizer use and promote sustainable agricultural practices.
Share your experiences with nitrogen management in the comments below. Explore our advanced guide to sustainable agriculture for more in-depth information. Contact our experts for a consultation on optimizing nitrogen fertilizer use in your specific situation.
